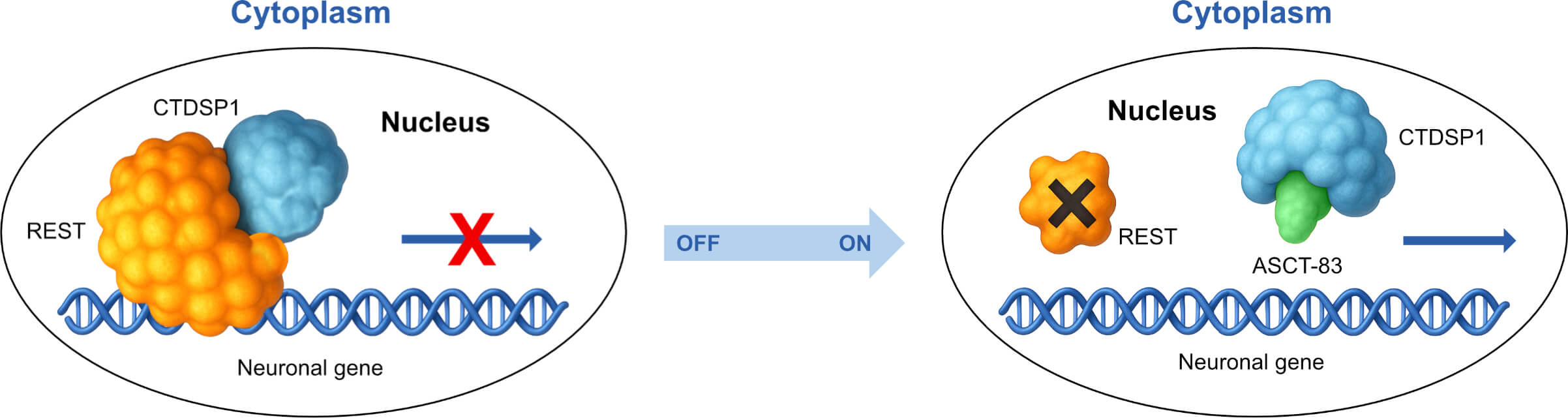
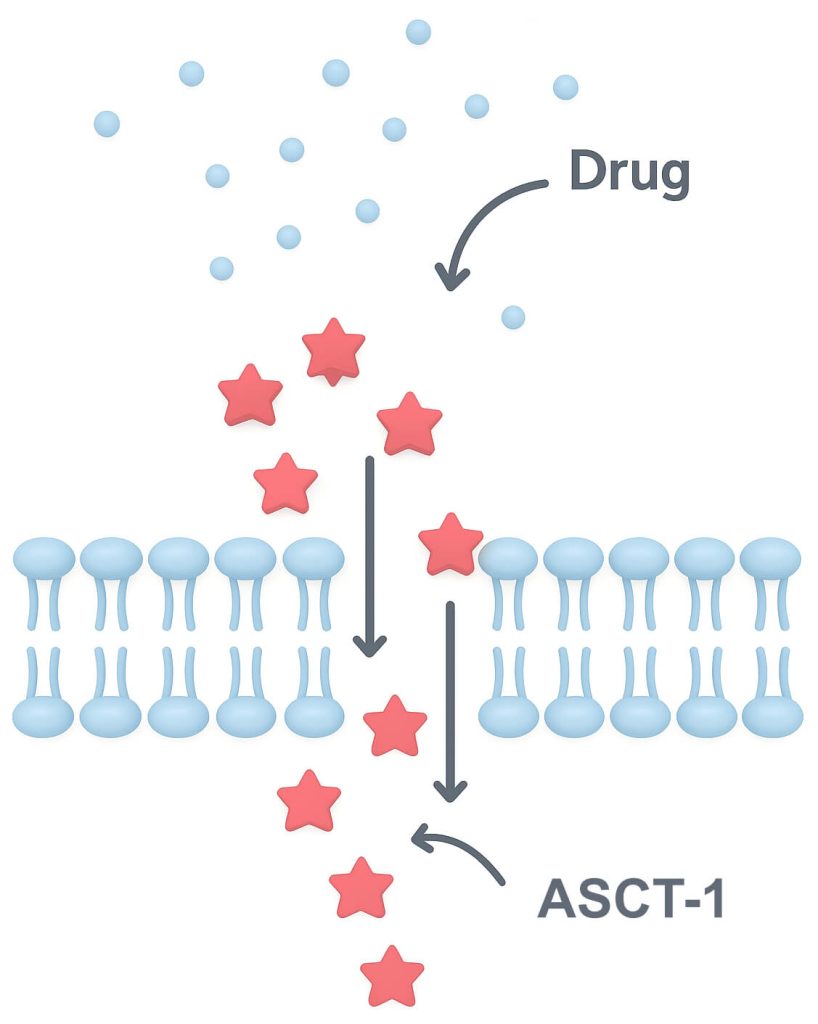
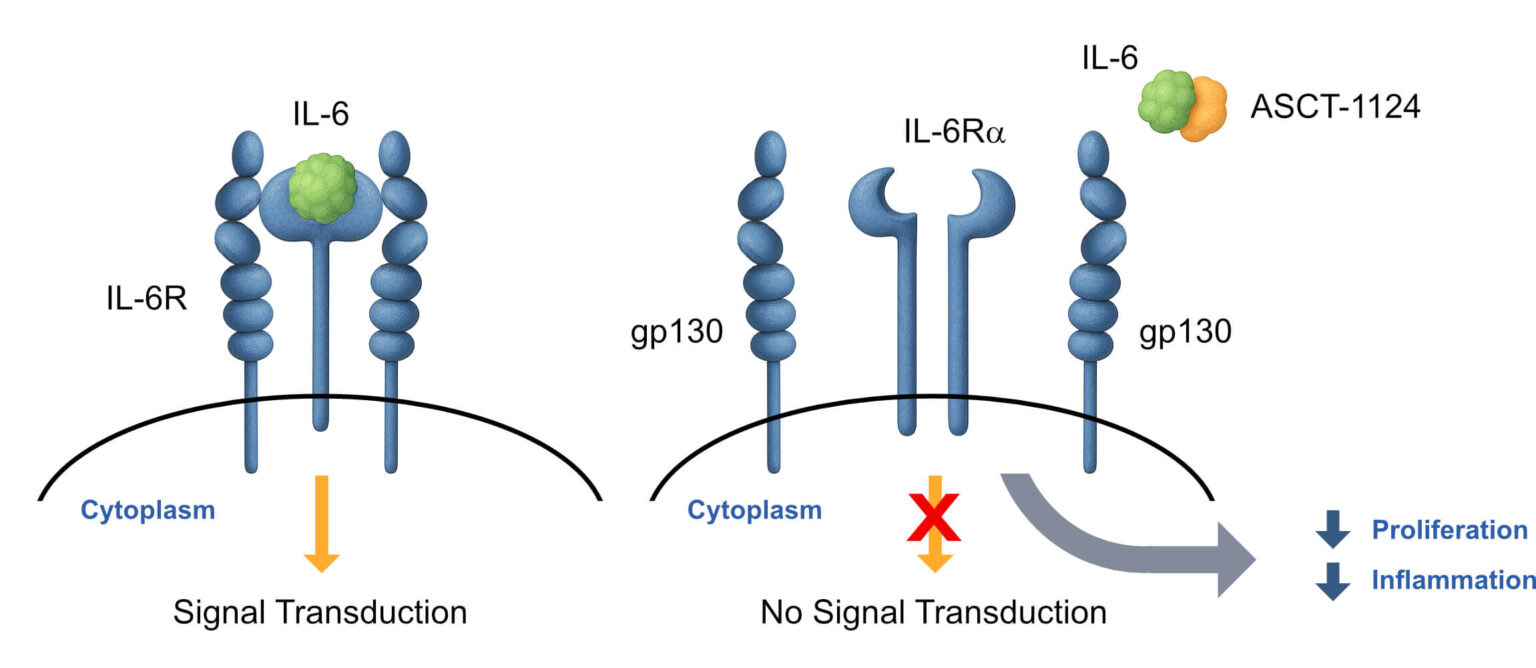
Increased ASCT-456 REST antagonist activity from 64% to 100%
Terms and Conditions
Alcamena Website Terms and Disclaimers:
Terms and Conditions
- Acceptance of Terms
By accessing and using the Alcamena website (“Site”), you agree to be bound by these Terms and Conditions (“Terms”). If you do not agree, please discontinue use immediately.
- Purpose of the Site
The Site provides information about Alcamena, its PepGenesis™ platform, research programs, and corporate initiatives. Content is for informational purposes only and does not constitute medical, legal, or investment advice.
- Intellectual Property
- All content, including text, graphics, logos, icons, images, and software, is the property of Alcamena or its licensors.
- You may not copy, reproduce, distribute, or create derivative works without prior written consent.
- Trademarks such as PepGenesis™ are proprietary and protected under applicable law.
- Use of Information
- You may view, download, or print materials for personal, non-commercial use only.
- Any unauthorized use may result in termination of access and potential legal action.
- No Medical Advice
- The Site may reference scientific research, therapeutic areas, or clinical programs.
- This information is not intended to replace professional medical consultation.
- Always seek advice from qualified healthcare providers regarding medical conditions or treatments.
- Investigational Products Disclaimer
- Pipeline assets and programs described on the Site are investigational and have not been approved by the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), or any other regulatory authority.
- Safety and efficacy have not been established outside of controlled clinical trials.
- Nothing on the Site should be construed as a promotion or solicitation for unapproved products.
- Third-Party Links
- The Site may contain links to third-party websites.
- Alcamena is not responsible for the content, accuracy, or practices of external sites.
- Accessing third-party links is at your own risk.
- Privacy
- Use of the Site is subject to Alcamena’s Privacy Policy, which explains how data may be collected and used.
- By using the Site, you consent to the practices described in the Privacy Policy.
- Disclaimers
- The Site is provided “as is” without warranties of any kind, express or implied.
- Alcamena does not guarantee that the Site will be error-free, uninterrupted, or free of harmful components.
- Alcamena disclaims liability for reliance on Site content.
- Forward-Looking Statements: Certain statements may be forward-looking and involve risks and uncertainties. Actual results may differ materially. Alcamena undertakes no obligation to update such statements.
- Limitation of Liability
- To the fullest extent permitted by law, Alcamena shall not be liable for damages arising from use of the Site, including indirect, incidental, or consequential damages.
- This includes damages related to reliance on information, loss of data, or inability to access the Site.
- Indemnification
You agree to indemnify and hold harmless Alcamena, its affiliates, officers, and employees from claims, liabilities, or expenses arising from your use of the Site or violation of these Terms.
- Governing Law
These Terms are governed by and construed under the laws of the State of New Jersey, United States, without regard to conflict of law principles.
- Changes to Terms
Alcamena reserves the right to update or modify these Terms at any time. Changes will be posted on this page with an updated “Last Revised” date. Continued use of the Site constitutes acceptance of revised Terms.
- Contact Information
For questions about these Terms, please contact:
Alcamena Stem Cell Therapeutics, LLC
1450 South Rolling Road, Suite 4.069
Baltimore MD, 21227-3867
Email: info@alcastem.com
Forward-Looking Statements
This website may contain forward-looking statements within the meaning of applicable securities laws. These statements are based on Alcamena’s current expectations, forecasts, and assumptions and involve risks and uncertainties that could cause actual outcomes to differ materially from those expressed or implied.
Forward-looking statements may include, but are not limited to:
- Development plans for the PepGenesis™ platform and other investigational programs
- Anticipated clinical trial designs, timelines, and regulatory submissions
- Potential therapeutic benefits, safety, or efficacy of investigational assets
- Strategic partnerships, collaborations, or business development initiatives
- Financial projections, operational milestones, and future growth opportunities
Such statements are subject to risks and uncertainties, including scientific, regulatory, operational, and market factors. Actual results may differ materially due to risks inherent in drug development, regulatory review processes, manufacturing, commercialization, and other factors beyond Alcamena’s control.
Alcamena undertakes no obligation to update or revise forward-looking statements, whether as a result of new information, future events, or otherwise, except as required by law.
Clinical Trial Information Disclaimer
Information regarding clinical trials provided on this Site is intended for general informational purposes only.
- Clinical trial listings may not be comprehensive or up to date.
- Participation in any clinical trial is subject to eligibility criteria, informed consent, and approval by regulatory and ethical review boards.
- Nothing on this Site should be construed as a solicitation to participate in a clinical trial.
- Individuals interested in clinical trials should consult with qualified healthcare providers and review official trial registries (e.g., ClinicalTrials.gov) for authoritative information.
“Privacy Policy”
Here’s a unified Privacy Policy for Alcamena’s website, now including a HIPAA-specific disclaimer to clarify that no Protected Health Information (PHI) is collected or stored. This version is comprehensive and harmonized for biotech corporate use: