1450 South Rolling Road, Suite 4.069, Halethorpe, MD 21227
Questions? We're here to help.
Discover how our advanced protein engineering solutions can support your next breakthrough—let’s connect.
1450 South Rolling Road, Suite 4.069, Halethorpe, MD 21227
1-650-307-4940
a.pisarchik@alcastem.com
Discover how our advanced protein engineering solutions can support your next breakthrough—let’s connect.
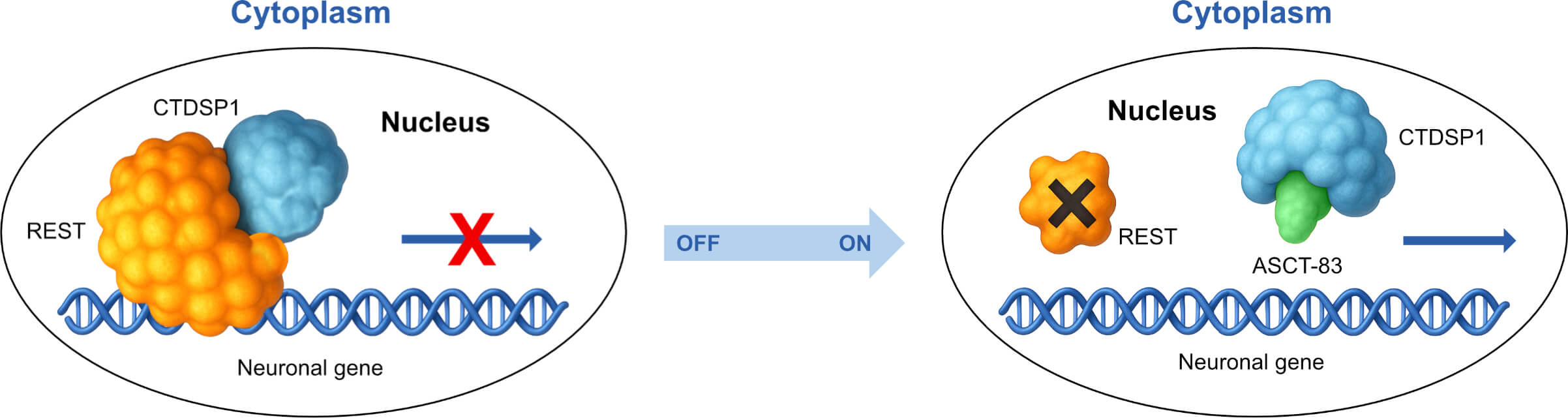
CTDSP1: C-terminal domain small phosphatase 1; REST: RE1 silencing transcription factor
ASCT-83 is a clinical stage first-in-class drug that antagonizes CTDSP1 and REST, which repress neuronal genes1. Prior to ASCT-83, CTDSP1 and REST were considered undruggable because they lack adequate binding pockets for small molecules and are localized in the nucleus, making them inaccessible to antibodies. CTDSP1 and REST are absent or only express at low levels in neurons and pancreatic beta cells (functionally similar to neurons). However, sugary and processed foods, or nerve injury can result in excessive expression in these tissues. High expression levels cause dysglycemia, neuropathy, and in the case in traumatic nerve and brain injury, an incomplete recovery. Additionally, brain cancers, like glioblastoma, have high CTDSP1 and REST levels. The published literature and our data strongly support that antagonizing CTDSP1 and REST improves the outcome of metabolic disorders, nerve injury, and brain cancer2-7.
Important for safety, most REST targets are transcriptionally unresponsive, because the basal organization of chromatin does not permit the epigenetic “writing” of a new set of instructions. However, ASCT-83 mediated-interference of REST chromatin binding in injured or diseased cells permits the re-establishment of durable chromatin state, restoring normal gene expression, thereby addressing the underlying cause of metabolic and/or neurologic symptoms, with a low potential for side effects.
Alcamena has received a “may proceed” letter from the Food and Drug Administration (FDA) to conduct a single and multiple ascending dose study in health human subjects to assess the tolerability and pharmacokinetics of ASCT-83 following daily subcutaneous administration.
Scientific references:

Scientific references:
Scientific references:
Scientific references:
Scientific references:
Scientific references:
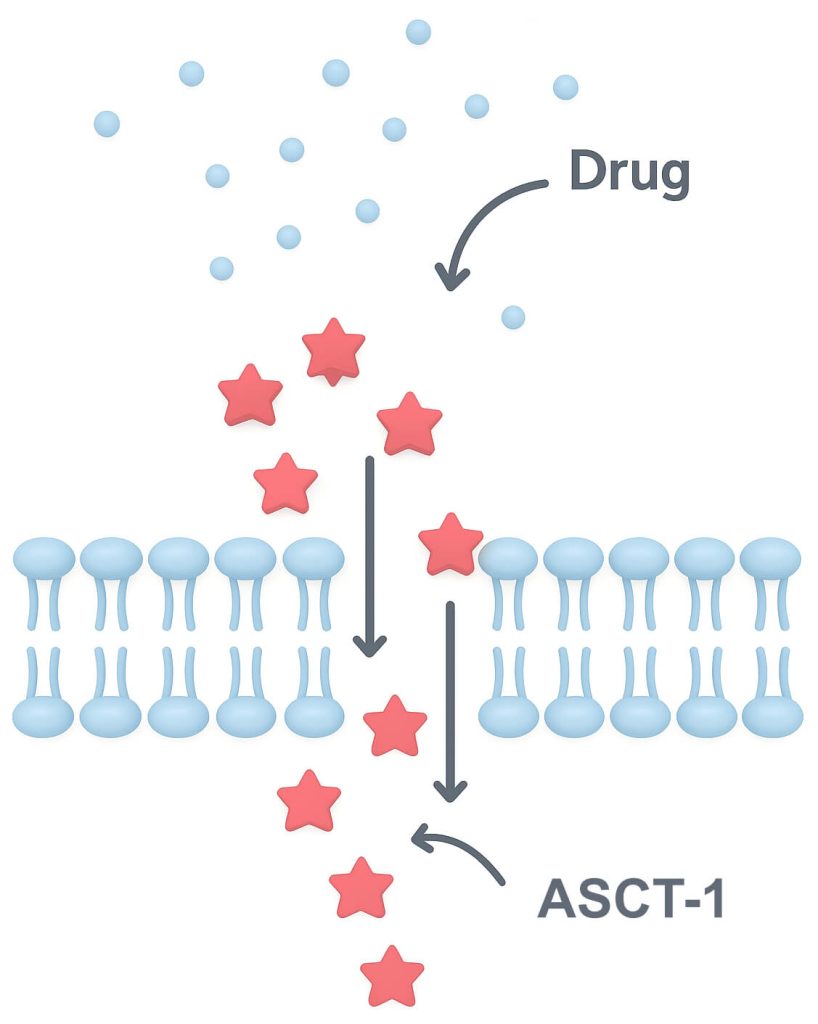
Mechanism: ASCT-1 is engineered to optimize the delivery of small molecules, peptides, antibodies and oligonucleotides.
With high membrane affinity and efficient membrane permeability, it acts as a “molecular doorman”, facilitating transmembrane passage and improving systemic uptake.
Increased ASCT-456 REST antagonist activity from 64% to 100%
Increases ASCT-456-induced U87 GBM cell death from 63% to 100%.
Increases ASCT-456-induced U251-TMZ resistant GBM cell death from 0 to 76%
ASCT-1 exhibits no off-target effect on its own.
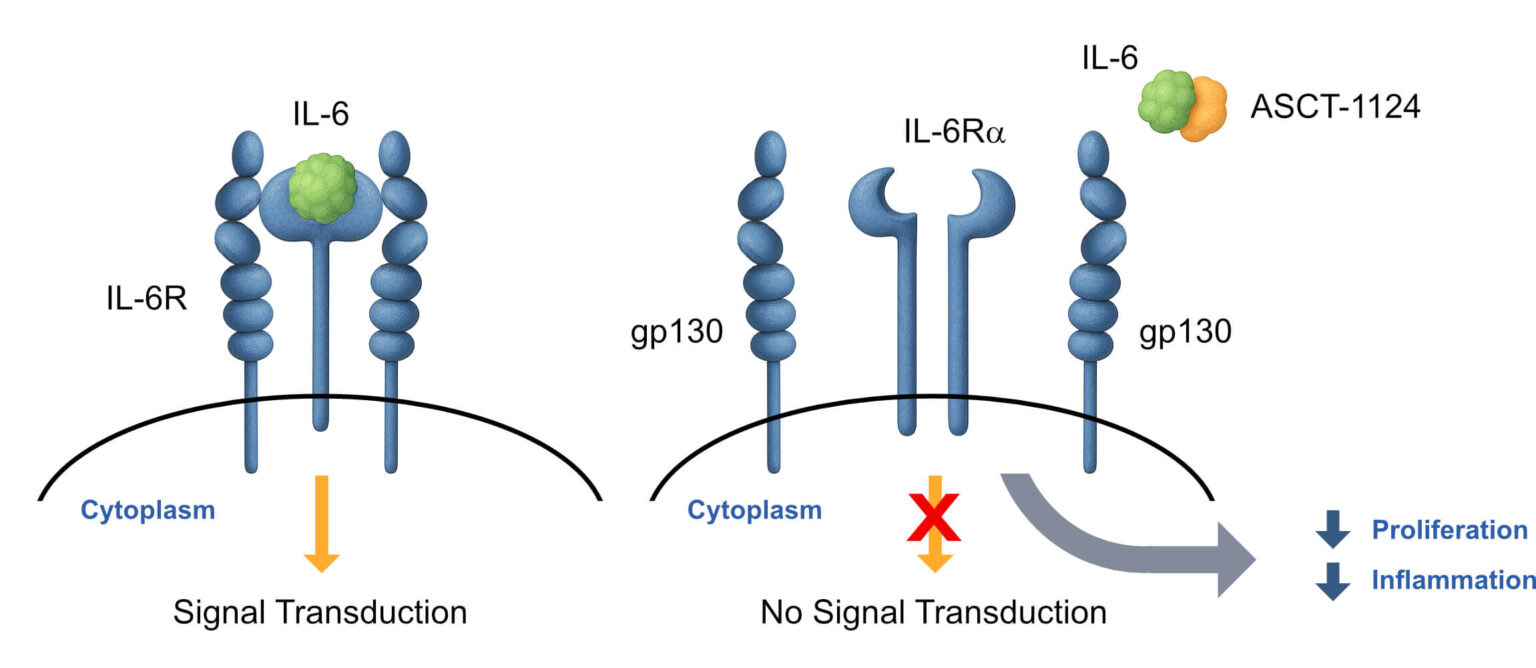
Mechanism: A first-in-class topical peptide that binds directly to IL-6, blocking proliferation and inflammation signals. This offers a localized treatment, minimizing systemic side effects.
ASCT-1124 is a topical peptide that delivers biologic-level potency directly only to the disease site, avoiding systemic toxicity.
ASCT-1124 dose-dependently decreases proliferation of skin cells (keratinocytes) from 140% with IL-6 alone to zero in the presence of ASCT-1124.
40% reduction in skin thickness, 60% reduction in serum IL-6, and 75% reduction in serum IFN-γ in a humanized IMQ mouse model.
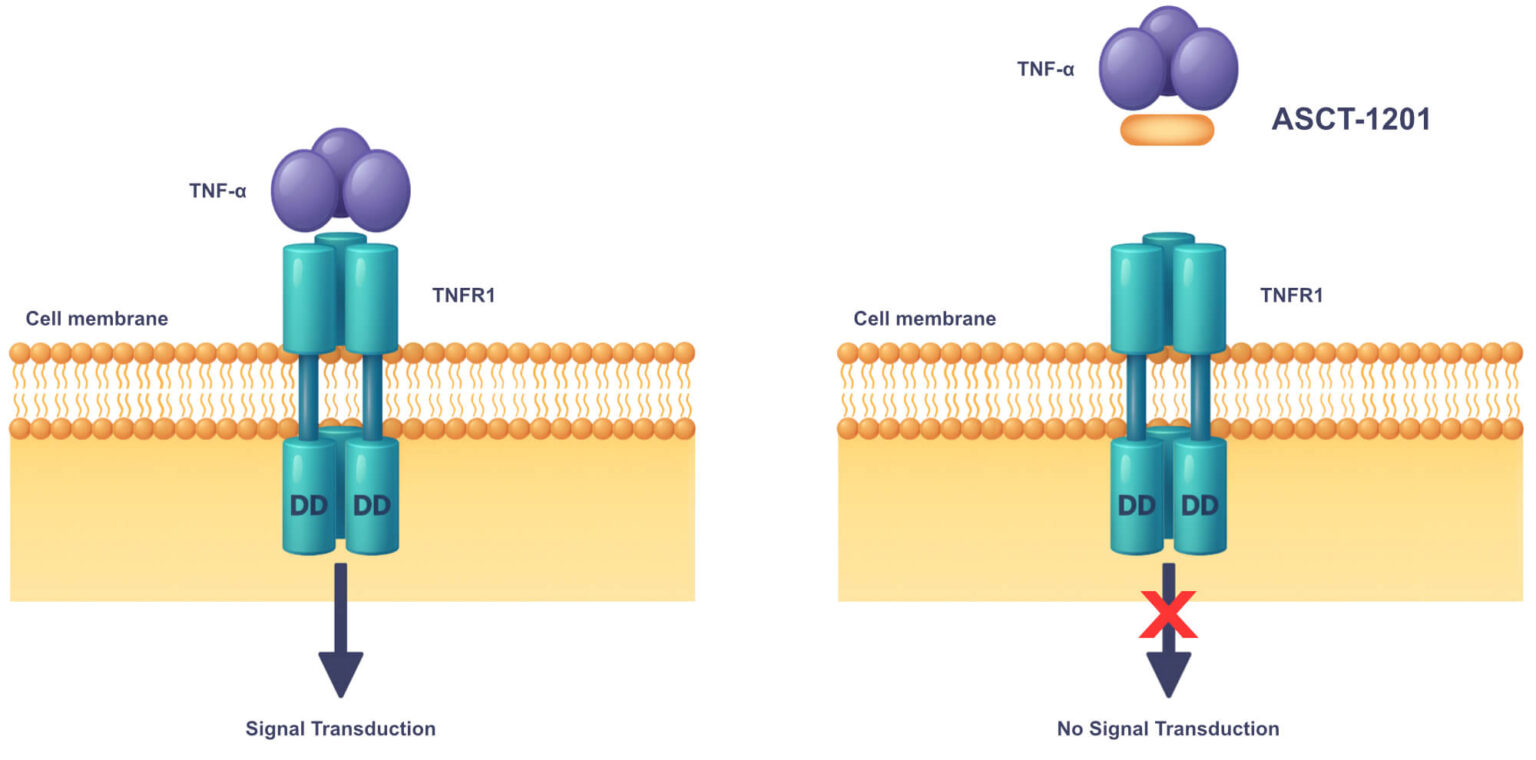
Pathophysiology: In both rheumatoid arthritis (RA) and psoriatic arthritis (PsA), elevated TNF-α in the synovial fluid and tissue causes joint destruction.
ASCT-1201 is a once-monthly intra-articular (IA) injection that treats only the inflamed joint, eliminating systemic immunosuppression.
Low nanomolar affinity with no off-target activity.
>40% Reduction in TNF-α signaling in a reporter assay, confirming potent target engagement.
Model: TNF reporter strain