Increased ASCT-456 REST antagonist activity from 64% to 100%
Leadership

Edmund Nesti, PhD
Chief Executive Officer
Edmund Nesti leads Alcamena with a clear mission: to disrupt drug discovery and unlock the “undruggable” universe. Dr. Nesti’s leadership is focused on addressing large, unmet medical needs by pioneering transformative new therapeutic modalities.
Under Dr. Nesti’s direction, Alcamena has developed its Supernatural Peptides™ platform. This platform integrates a GenAI-Guided Directed Evolution engine, which has proven its ability to move assets from target nomination to Investigational New Drug (IND) application-ready status with unprecedented speed. This is validated by the company’s lead program, ASCT-83, which became IND-ready in under 12 months and is now in Phase 1 clinical development.
Dr. Nesti’s strategic vision is grounded in deep regulatory and scientific expertise. Dr. Nesti’s background includes experience at the U.S. Food & Drug Administration (FDA) and the Howard Hughes Medical Institute (HHMI). Dr. Nesti’s has personal experience with more than 100 INDs and 6 FDA New Drug Application (NDA) approvals, guiding the team that has collective experience with over 1,000 INDs.
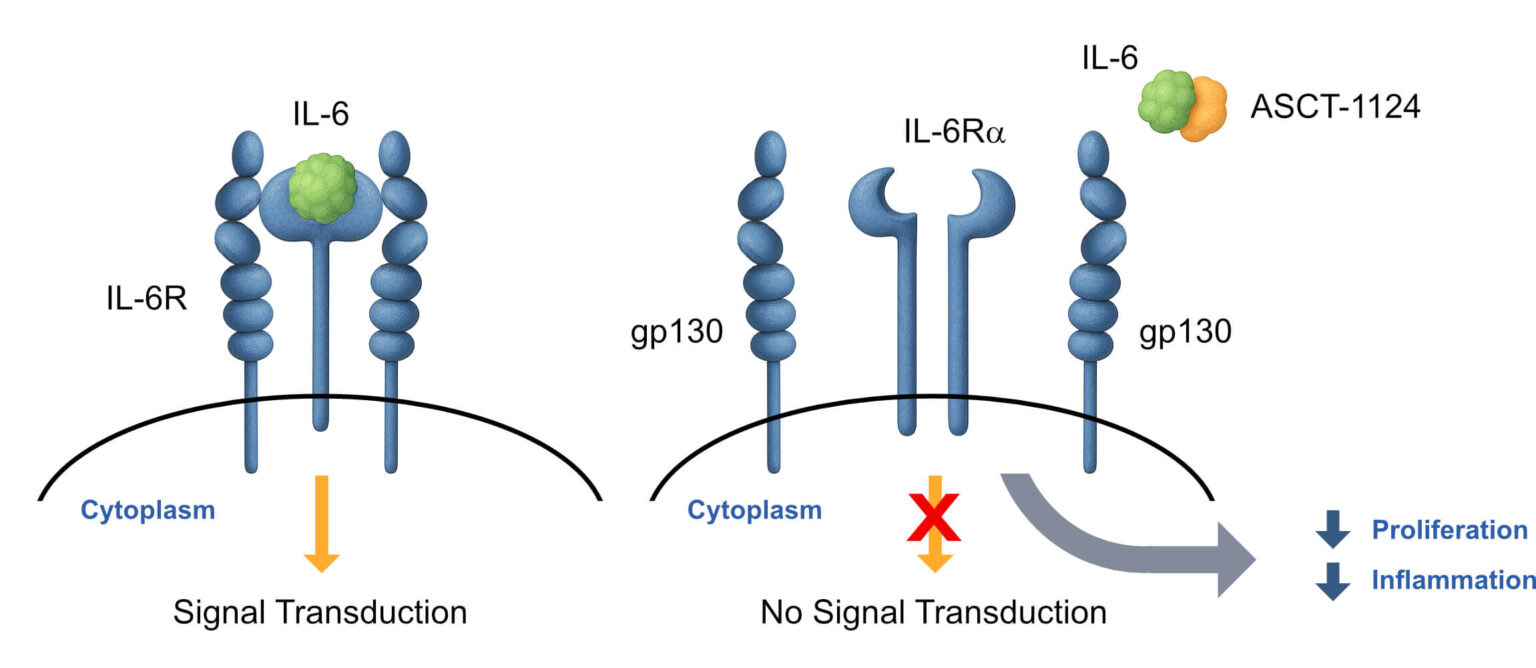
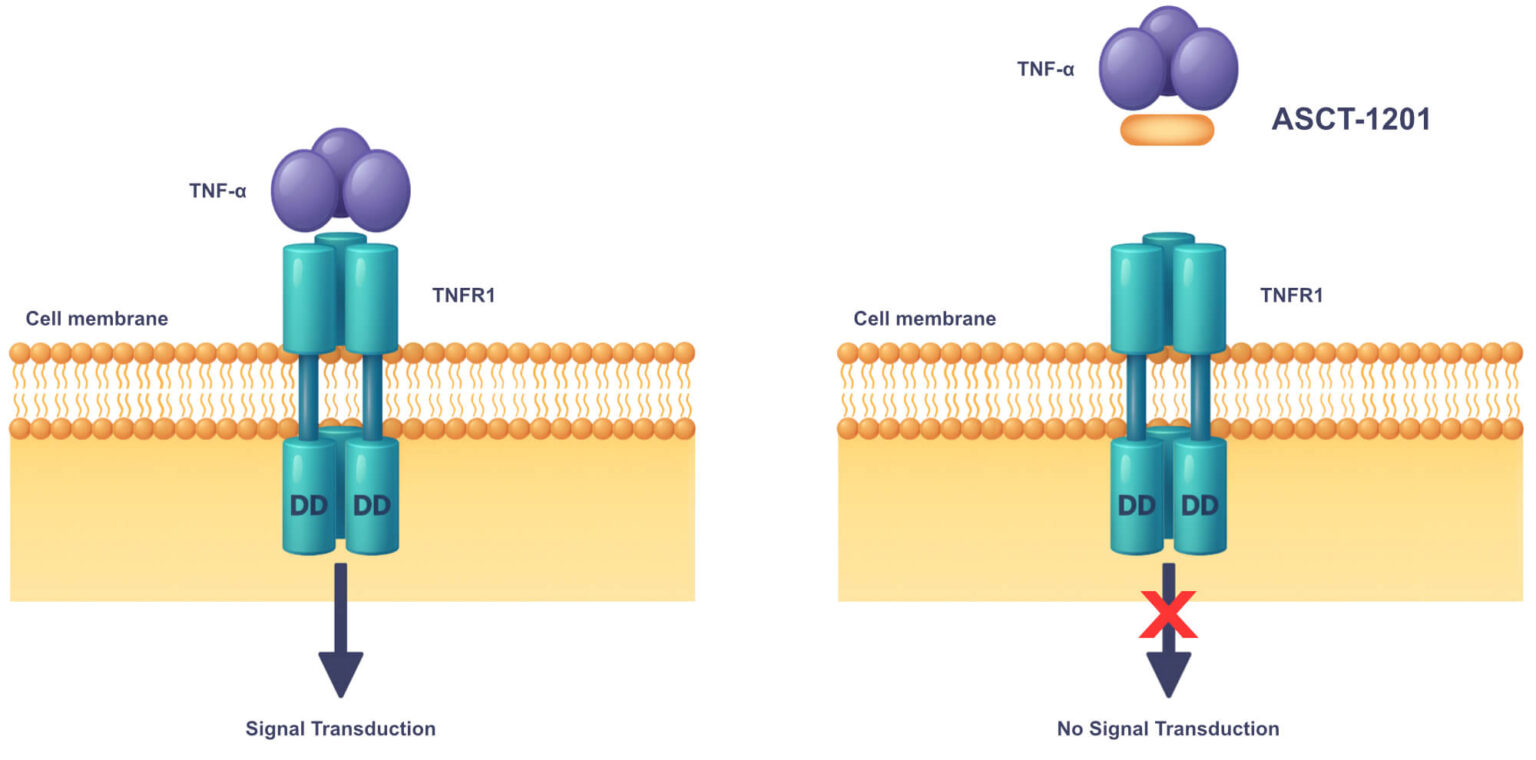
Dr. Nesti is now actively seeking partners to accelerate Alcamena’s robust pipeline, specifically for the co-development of ASCT-1124 (IL-6 antagonist) and ASCT-1201 (TNF-α antagonist), to deliver these promising, differentiated therapies to patients worldwide.

Alex Pisarchik, PhD
Chief Operating Officer and Director of Protein Engineering
Alex Pisarchik is the principal architect of Alcamena’s Supernatural Peptides™ technology engine. He leverages over a decade of protein engineering expertise from industry pioneers like DuPont, Genencor, Arzeda, and ConstructiveBio to drive the company’s scientific operations.
Dr. Pisarchik led the development of Alcamena’s proprietary DMTA GenAI-guided directed evolution platform. This system, integrating PepFusion™ tunable libraries and the PepGenesis™ AI-design engine, is precisely what enables Alcamena to target the “undruggable” universe, particularly complex protein-protein interactions.
The power of this platform is validated by Alcamena’s lead asset, ASCT-83, which advanced from target nomination to an IND-ready candidate in under 12 months. Now in Phase 1 clinical development, ASCT-83 has demonstrated a compelling preclinical profile, including:
- Potent appetite suppression and significant weight loss in canine models
- Robust pH stability (from pH 1-10)
- Confirmed oral (PO) and intranasal (IN) delivery potential
- Strong neuroregenerative and neuroprotective activity
Dr. Pisarchik is leading them to apply this same validated, high-speed platform to propel the assets at the center of this co-development proposal—ASCT-1124 (IL-6) and ASCT-1201 (TNF-α)—toward their 12-month IND-enabling milestones.

Noreen Gervasi, MD, PhD
Director of Neurologic Drug Development
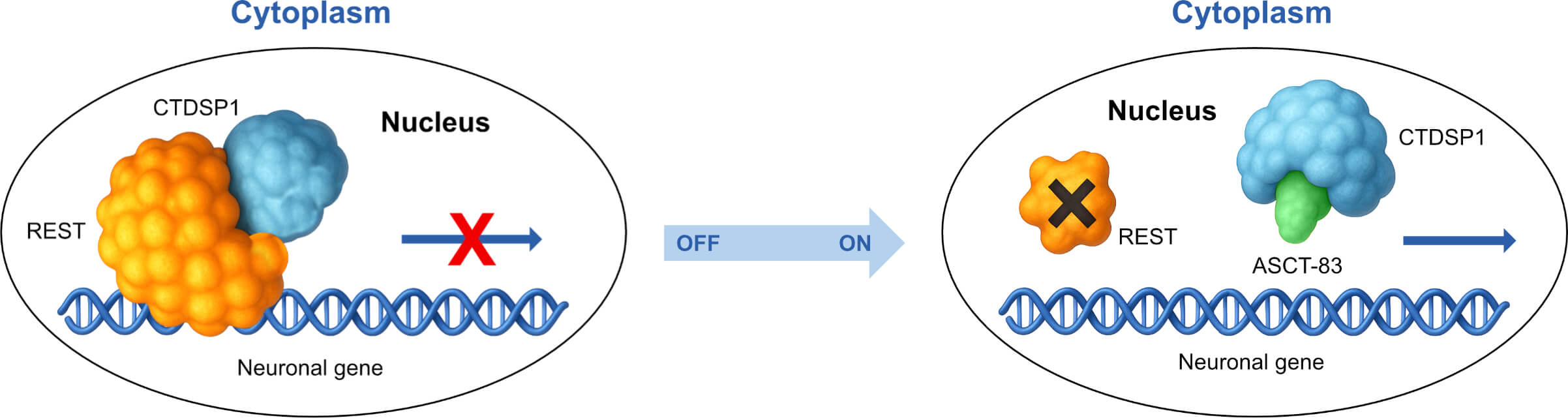
Noreen Gervasi leads Alcamena’s neurologic drug development, leveraging her deep expertise from the National Institutes of Health (NIH) and the Uniformed Services University (USU) to translate complex neuroscience into viable therapeutics. Dr. Gervasi’s work is centered on Alcamena’s lead Phase 1 asset, ASCT-83, a first-in-class antagonist targeting the “undruggable” REST:CTDSP1 protein-protein interaction. Her nonclinical development oversight has been pivotal in validating the Supernatural Peptides™ platform by demonstrating ASCT-83’s powerful and diverse activities.
Under her direction, ASCT-83 has produced a compelling, data-driven preclinical package for its neurological indications, including:
- Neuroregeneration: Dose-dependent increase in neurite outgrowth and promotion of neuronal differentiation via the upregulation of key markers (Tuj1/MAP2).
- Neuroprotection: Prevention of excitotoxicity and “brain death” in glutamate-injured hippocampal tissue.
- Neuropathic Pain: Significant alleviation of mechanical and thermal pain sensitivity in preclinical models.
- CNS Injury Recovery: Improvement of cognitive and motor function following brain and peripheral nerve injuries.
- Neuro-Oncology: Reduction in cell viability across glioblastoma (GBM) cell lines.
Dr. Gervasi’s work on ASCT-83 has provided the critical in vivo validation for the entire Alcamena platform, de-risking the technology that underpins the ASCT-1124 (IL-6) and ASCT-1201 (TNF-α) co-development programs.

Leon Nesti, MD, PhD
Chief of Clinical and Experimental Medicine
Leon Nesti brings over 20 years of experience as a U.S. Army orthopaedic hand surgeon and scientist to lead Alcamena’s clinical and translational efforts. Dr. Nesti’s firsthand experience treating trauma-induced pain and neuropathy informs his commitment to developing transformative peptide therapeutics.
Dr. Nesti’s distinguished career includes combat casualty care at Walter Reed, a postdoctoral fellowship at the NIH, and board certification in hand and upper extremity surgery. Dr. Nesti research on trauma-induced gene expression and progenitor cell activity directly informs Alcamena’s pipeline and platform strategy.
With deep expertise in regenerative medicine and translational neuroscience, Dr. Nesti bridges clinical insight and scientific innovation to accelerate the development of high-impact therapies for patients worldwide.

Shawn Chen, MBA
Chief Financial Officer
Shawn Chen brings over 25 years of financial leadership in government contracting, with deep expertise in growth strategy, operational efficiency, and regulatory compliance. Mr. Chen is the founder of Quantum Consulting Solutions, LLC, where Mr. Chen has advised clients on profitability, accounting systems, and financial health—serving as a trusted liaison with auditors, banks, and regulatory agencies.
Mr. Chen has held CFO roles at Williams Adley & Co, LLP, Thomas & Herbert Consulting, and System High Corporation, and served as VP of Client Services at NeoSystems Corporation. Mr. Chen experience spans DCAA compliance, incurred cost submissions, and optimizing cash flow and financial systems.
Mr. Chen earned his MBA from the Foster School of Business at the University of Washington.