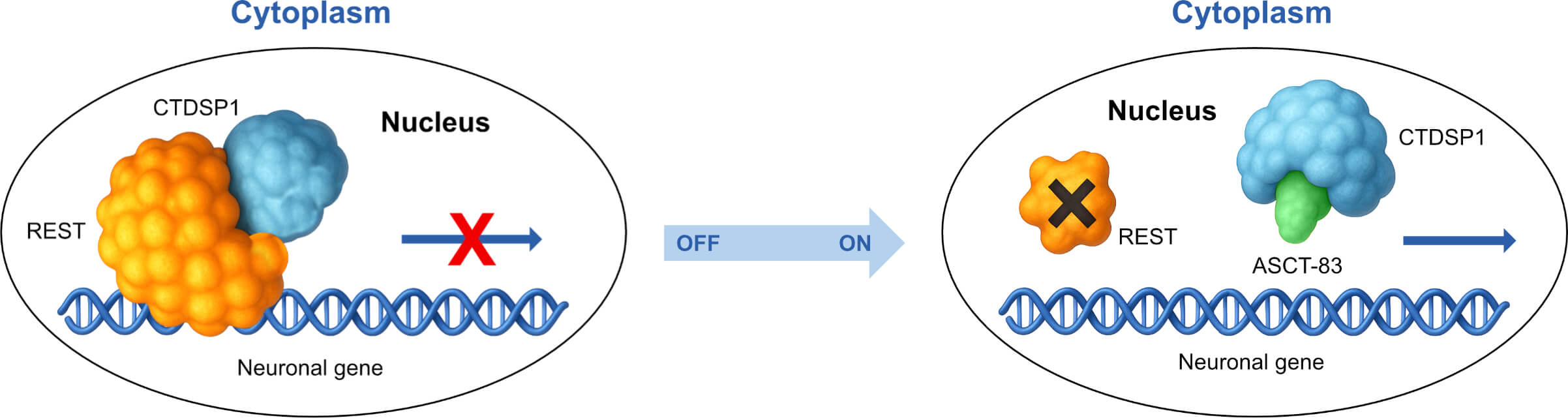
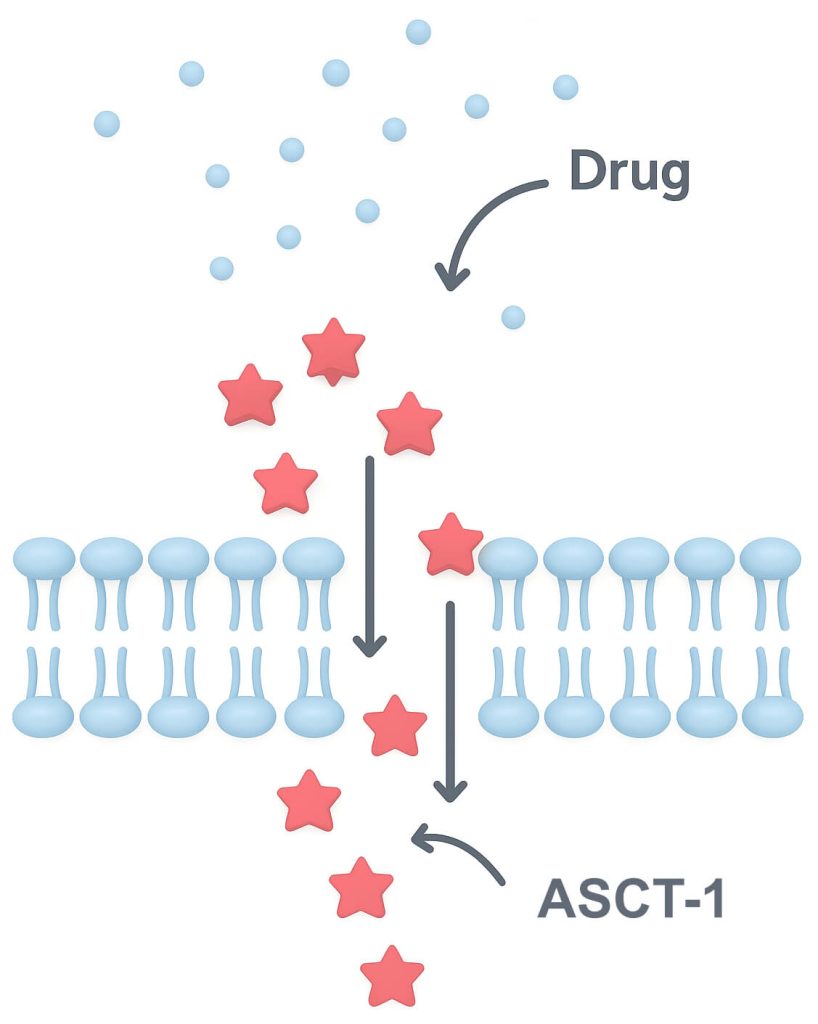
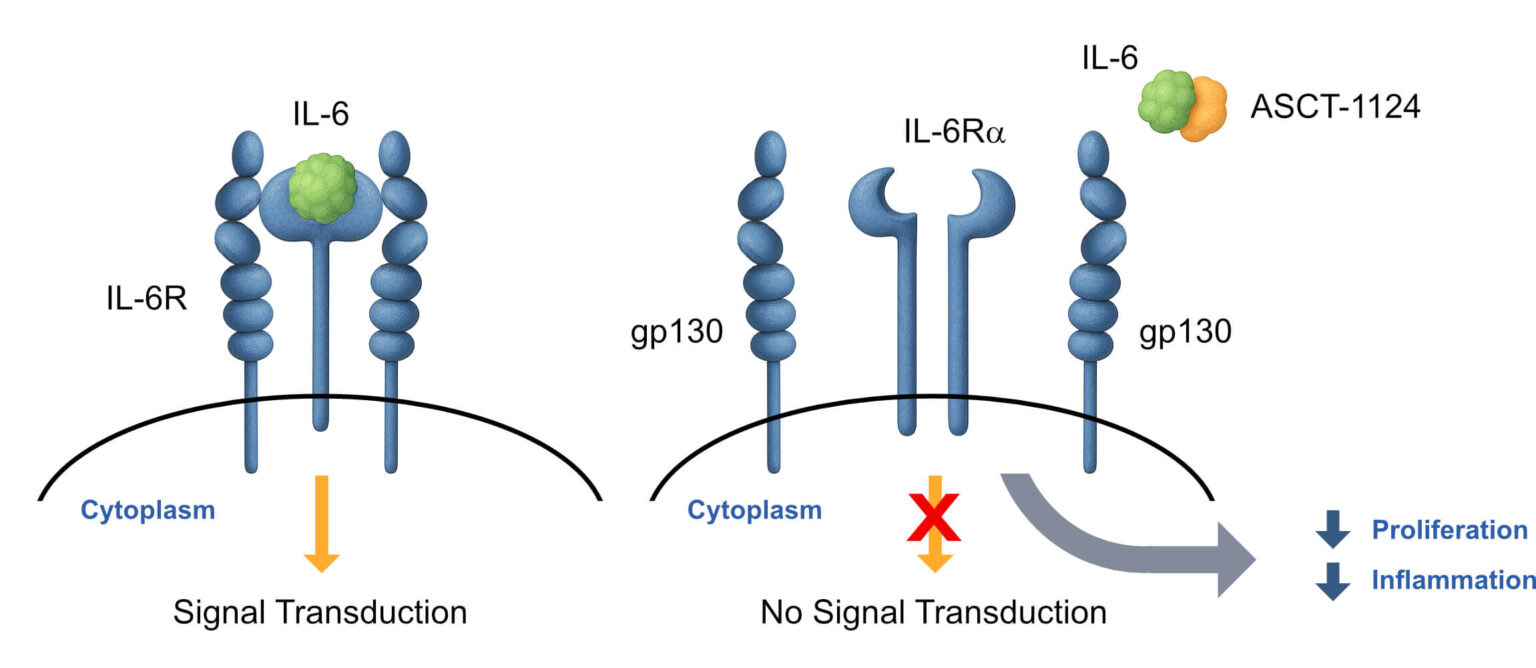
Target
Identification
Partnering

Expanding the Horizon: Partner with Alcamena
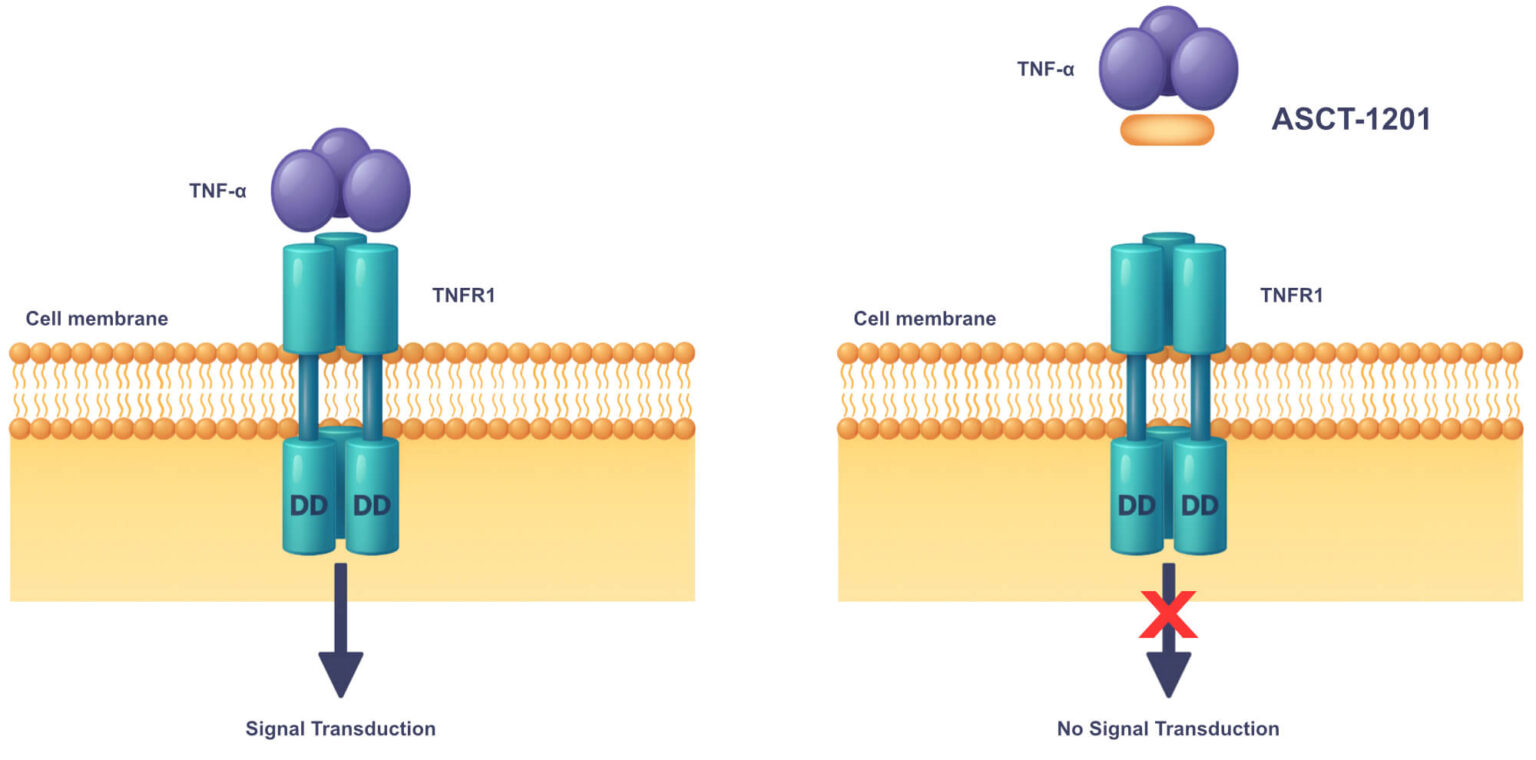
Asset Partnership: Beyond ASCT-83, our robust pipeline includes promising pre-clinical assets targeting severe dermatological conditions and autoimmune diseases, such as ASCT-1124 and ASCT- 1201. We are actively seeking strategic partnerships to accelerate the development and impact of our Supernatural Peptides™ across a broad range of therapeutic areas.
Personalized Medicine / De Novo Drug Development Partnership: We will partner with you to develop a novel drug for any target. This program is ideally suited for personalized medicine projects to address life threatening health conditions, that require a rapid therapeutic response.
Flexible Partnerships to Accelerate Breakthrough Therapeutics
Target Discovery → Supernatural PeptidesTM → Clinical Development
Partnering Opportunities:
Alcamena offers:
Rapidly advance new medicines into the clinic - together
Goal: 1-3 Years

Project Example: Personalized Therapeutic Development
Developing a Single-Patient Phase 1/2 Study in One Year
Executive Summary
Why Partner with Alcamena?
The goal is not just about finding new binders. Instead, it is about rapidly evolving leads into potent, tissue-targeted, and translation-read medicines.
Differentiation
Validated Platform
Let’s Unlock the Therapeutic Potential of Supernatural Peptides™ — Together